Specific tests for Alzheimer’s disease (AD) diagnosis are currently unavailable, despite AD being the leading cause of dementia. One hallmark of AD progression is the aggregation of tau proteins into paired helical filaments and neurofibrillary tangles, which is accelerated by the hyperphosphorylation of Tau proteins. However, the mechanism by which the hyperphosphorylated tau accelerates protein aggregation is not completely understood. Furthermore, detecting and disrupting such aggregated forms through the blood-brain barrier (BBB) remains a significant bottleneck in developing AD diagnostics and therapeutics. At the same time, quantum dots (QDs) have shown tremendous potential in penetrating the BBB to diagnose brain cancer, as well as detecting and disrupting protein aggregates in other neurodegenerative diseases such as Parkinson’s disease. QDs are an attractive diagnostic material due to their fluorescence-emitting capabilities, nanoscale size that allows penetration of the BBB, chemical stability, solubility, and facile synthesis. However, QDs have not yet been assessed for their ability to detect and disrupt hyperphosphorylated tau tangles. Hence, the aims of this project are two-fold: 1) to unravel the mechanisms and energetic barriers of normal and hyperphosphorylated tau protein aggregation by building three-dimensional atomistic models of aggregated structures and performing classical and enhanced sampling molecular dynamics simulations on these models; 2) to predict the potential of QDs in binding to and disrupting hyperphosphorylated tau tangles though polarized ligand docking and free-energy calculations. Upon identification of potential QD-binding signatures, these QDs will be synthesized and tested in vitro and in vivo through collaborative efforts with the goal of translating this work into clinical diagnostic applications for AD in the future.
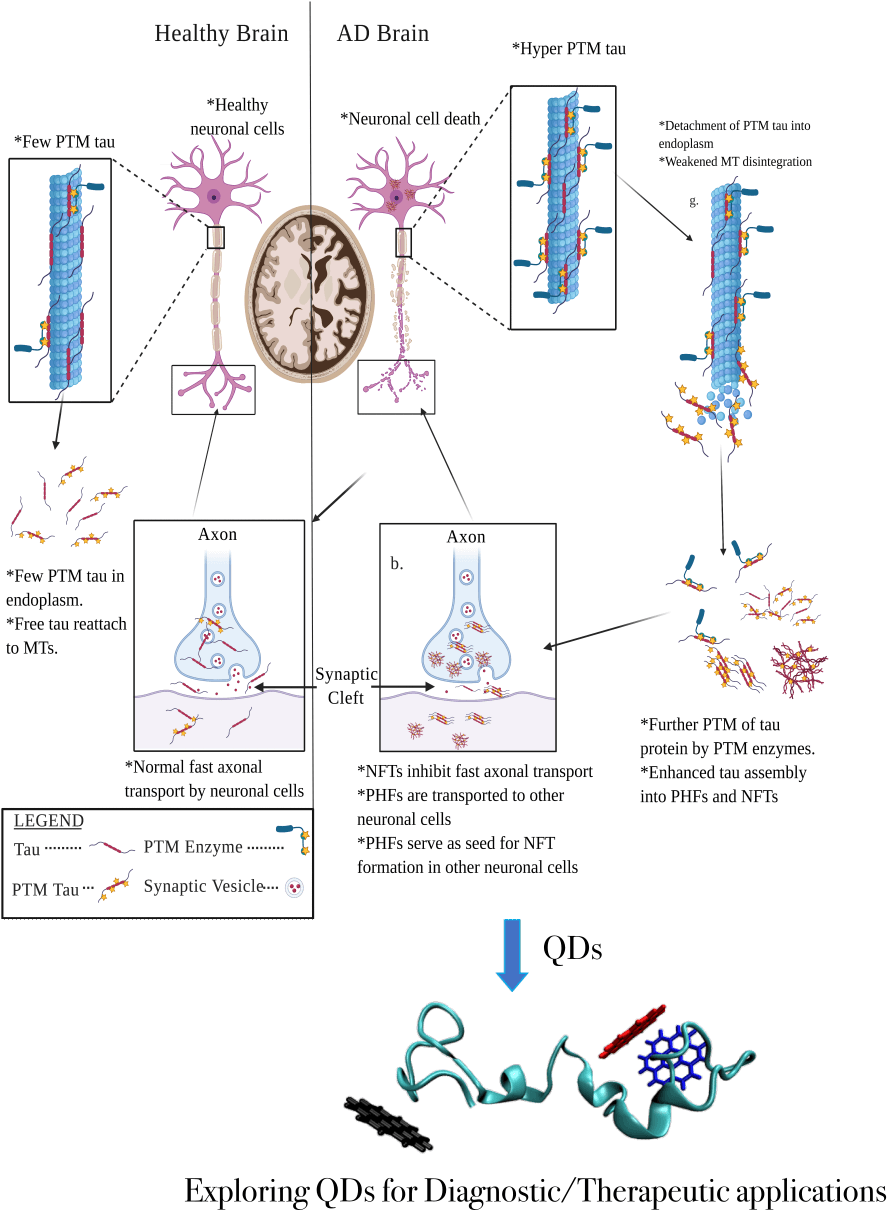
Figure 1. Microtubule-associated protein tau (MAPT) functions in the healthy brain (left) and a brain with Alzheimer’s disease (AD) (right). Self-association and excessive post-translational modifications of Tau proteins result in the formation of neurofibrillary tangles and cause neurodegeneration in AD patients. Targeting the tau aggregates using Quantum Dots could help develop potential diagnostics and/or therapeutics for AD.
Related Content
Novel High-Speed Receiver for Quantum Communication and Sensing
Summary An essential aspect of a quantum channel is the detection and analysis of quantum signals in the form of photons. For most free-space applications, the photons are polarization encoded, e.g. by assigning the ‘0’ to horizontally polarized photons and ‘1’ to vertically polarized photons. However, where the geometric reference is not constant at all […]
January 1, 2019

A Reformulation of Quantum Game Theory
Summary Classical game theory – conducted at the interface between economics and computer science – has found applications in topics ranging from networking and security to online markets. Despite over 20 years of research into connections between game theory and quantum information, we have yet to see any significant implications of quantum information when applied […]
April 1, 2020

Two-Dimensional Quantum Materials and Heterostructures
Two-dimensional (2D) layers just one atom thick can be stripped from certain materials, such as graphene.
June 1, 2017
Quantum Sensing Applications using Quantum Communication Technology
Summary The Quantum Encryption and Science Satellite provides a platform to develop and deploy quantum sensing and metrology via photonic channels. This project will build upon ‘free-space’ quantum communication technology and explore new approaches and methods to advance two primary applications: quantum-enhanced telescopes, and spectroscopic sensing for methane detection in the atmosphere. For the […]
December 8, 2018

