Specific tests for Alzheimer’s disease (AD) diagnosis are currently unavailable, despite AD being the leading cause of dementia. One hallmark of AD progression is the aggregation of tau proteins into paired helical filaments and neurofibrillary tangles, which is accelerated by the hyperphosphorylation of Tau proteins. However, the mechanism by which the hyperphosphorylated tau accelerates protein aggregation is not completely understood. Furthermore, detecting and disrupting such aggregated forms through the blood-brain barrier (BBB) remains a significant bottleneck in developing AD diagnostics and therapeutics. At the same time, quantum dots (QDs) have shown tremendous potential in penetrating the BBB to diagnose brain cancer, as well as detecting and disrupting protein aggregates in other neurodegenerative diseases such as Parkinson’s disease. QDs are an attractive diagnostic material due to their fluorescence-emitting capabilities, nanoscale size that allows penetration of the BBB, chemical stability, solubility, and facile synthesis. However, QDs have not yet been assessed for their ability to detect and disrupt hyperphosphorylated tau tangles. Hence, the aims of this project are two-fold: 1) to unravel the mechanisms and energetic barriers of normal and hyperphosphorylated tau protein aggregation by building three-dimensional atomistic models of aggregated structures and performing classical and enhanced sampling molecular dynamics simulations on these models; 2) to predict the potential of QDs in binding to and disrupting hyperphosphorylated tau tangles though polarized ligand docking and free-energy calculations. Upon identification of potential QD-binding signatures, these QDs will be synthesized and tested in vitro and in vivo through collaborative efforts with the goal of translating this work into clinical diagnostic applications for AD in the future.
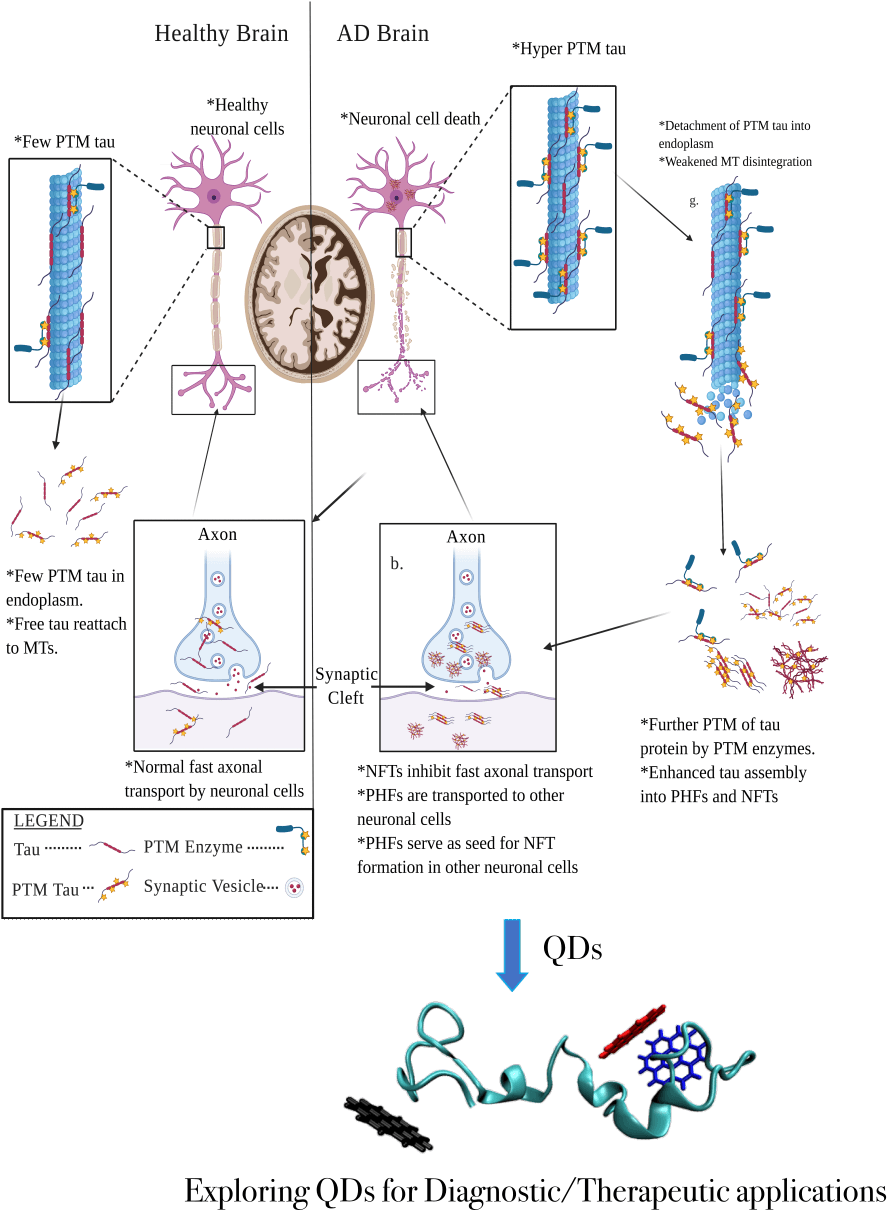
Figure 1. Microtubule-associated protein tau (MAPT) functions in the healthy brain (left) and a brain with Alzheimer’s disease (AD) (right). Self-association and excessive post-translational modifications of Tau proteins result in the formation of neurofibrillary tangles and cause neurodegeneration in AD patients. Targeting the tau aggregates using Quantum Dots could help develop potential diagnostics and/or therapeutics for AD.
Related Content

Towards large area, resonant quantum tunneling diodes by continuous Langmuir transfer of exfoliated 2D materials
Summary Atomically thin 2D materials constitute promising building blocks for quantum devices due to their exotic, layer-dependent electronic properties. The ability to stack these materials in alternating layers enables heterostructures to be built in almost limitless combinations and over small enough length scales to observe quantum phenomena. So far though, practical implementation of devices based […]
April 1, 2020

Free-space Polarization-selective Microcavity based on Chiral Metasurfaces
Summary Developing a new type of Fabry-Pérot cavity that allows improved control of the atoms’ emission into the cavity mode will result in enhancement of the efficiency and fidelity of quantum state transfer from photons to atoms and back. This in turn can be used to improve the performance of quantum networks and repeaters, as […]
September 19, 2019

Hybrid Quantum Materials towards Topological Quantum Computing
Summary Proximity engineered hybrid materials have shown promise for topological quantum information processing. This form of quantum computing provides a stable, error-tolerant approach for building scalable quantum information processors. Topological quantum computing relies on braiding non-Abelian particles, such as Majorana fermions, which do not exist in nature. One can however use materials engineering to […]
December 8, 2018


