Specific tests for Alzheimer’s disease (AD) diagnosis are currently unavailable, despite AD being the leading cause of dementia. One hallmark of AD progression is the aggregation of tau proteins into paired helical filaments and neurofibrillary tangles, which is accelerated by the hyperphosphorylation of Tau proteins. However, the mechanism by which the hyperphosphorylated tau accelerates protein aggregation is not completely understood. Furthermore, detecting and disrupting such aggregated forms through the blood-brain barrier (BBB) remains a significant bottleneck in developing AD diagnostics and therapeutics. At the same time, quantum dots (QDs) have shown tremendous potential in penetrating the BBB to diagnose brain cancer, as well as detecting and disrupting protein aggregates in other neurodegenerative diseases such as Parkinson’s disease. QDs are an attractive diagnostic material due to their fluorescence-emitting capabilities, nanoscale size that allows penetration of the BBB, chemical stability, solubility, and facile synthesis. However, QDs have not yet been assessed for their ability to detect and disrupt hyperphosphorylated tau tangles. Hence, the aims of this project are two-fold: 1) to unravel the mechanisms and energetic barriers of normal and hyperphosphorylated tau protein aggregation by building three-dimensional atomistic models of aggregated structures and performing classical and enhanced sampling molecular dynamics simulations on these models; 2) to predict the potential of QDs in binding to and disrupting hyperphosphorylated tau tangles though polarized ligand docking and free-energy calculations. Upon identification of potential QD-binding signatures, these QDs will be synthesized and tested in vitro and in vivo through collaborative efforts with the goal of translating this work into clinical diagnostic applications for AD in the future.
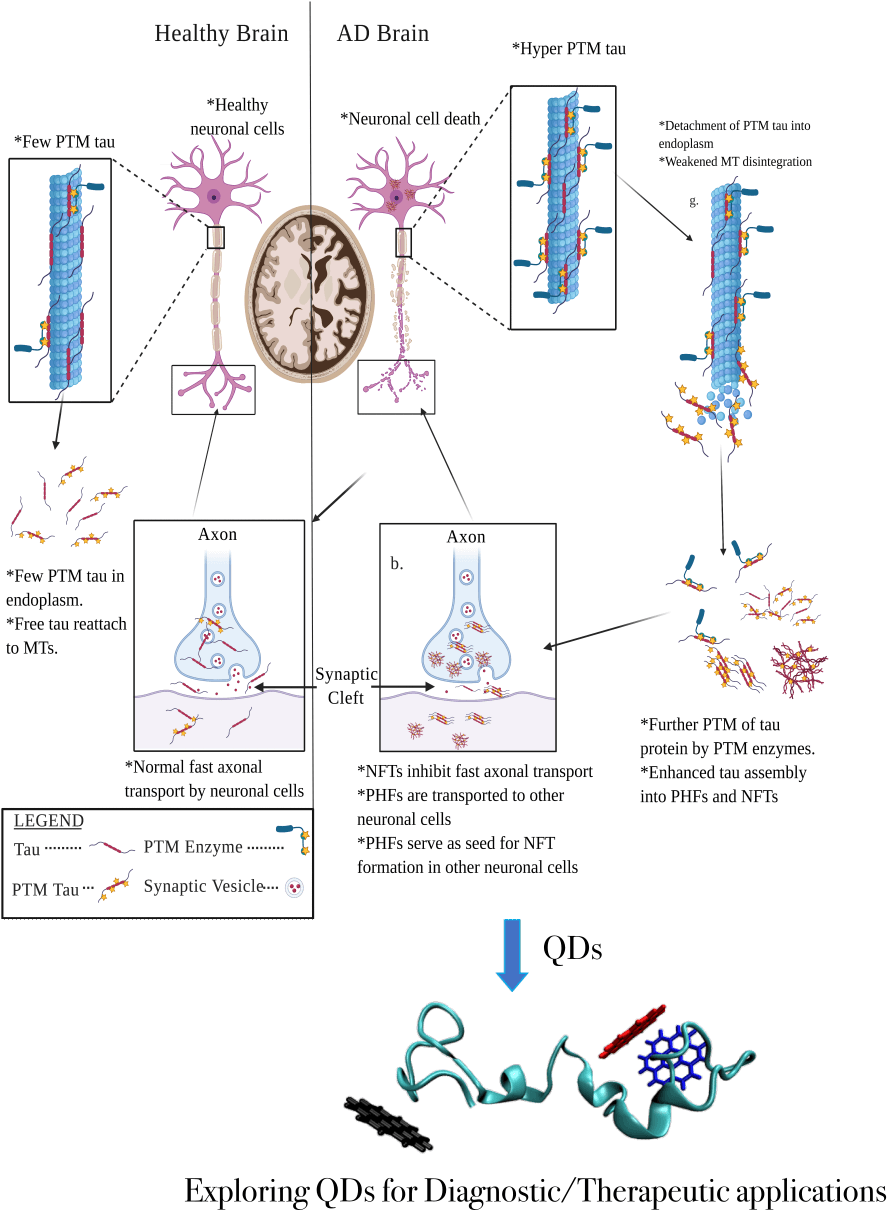
Figure 1. Microtubule-associated protein tau (MAPT) functions in the healthy brain (left) and a brain with Alzheimer’s disease (AD) (right). Self-association and excessive post-translational modifications of Tau proteins result in the formation of neurofibrillary tangles and cause neurodegeneration in AD patients. Targeting the tau aggregates using Quantum Dots could help develop potential diagnostics and/or therapeutics for AD.
Related Content
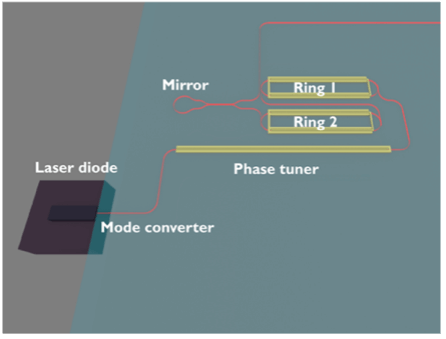
Visible wavelength external cavity diode lasers in photonic integrated circuits for atomic technologies
Atoms can be controlled by manipulating their internal states using agile, quiet and reliable laser sources. An external-cavity diode laser (ECDL) is a crucial enabling technology to realize such laser sources since it allows for the narrowing of the linewidth of a laser diode and precise tuning of the laser frequency. This project aims to […]
April 19, 2023

Hybrid Quantum Repeater based on Atomic Quantum Memories and Telecom Wavelength Entangled Photon-Pairs Generated from Semiconductor Nanowires
Summary Losses in physical channels, such as optical fibres, limit existing quantum communication systems to modest distance ranges. Since amplification of quantum signals is fundamentally not possible, we look to extend the range and functionality of these quantum channels by adding quantum memory nodes that can daisy-chain multiple lengths of quantum channels through entanglement […]
October 29, 2018
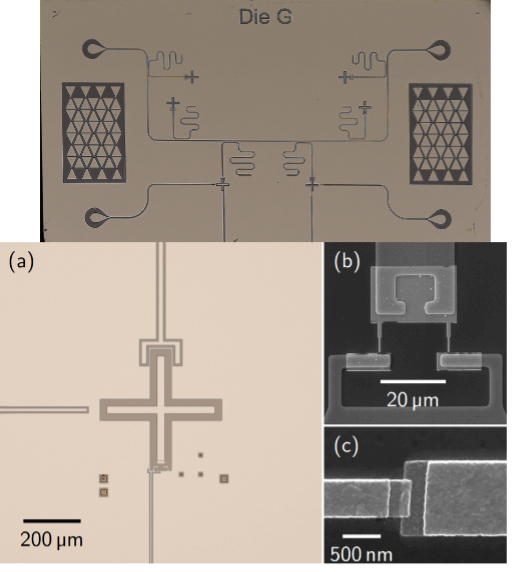
Building Blocks for Quantum Neuromorphic Computing: Superconducting Quantum Memcapacitors
Quantum neuromorphic computing (QNC) is a novel method that combines quantum computing with brain-inspired neuromorphic computing. Neuromorphic computing performs computations using a complex ensemble of artificial neurons and synapses (i.e., electrical circuits) to emulate the human brain. QNC may lead to a quantum advantage by realizing these components with quantum memory elements, or memelements, which […]
June 12, 2023
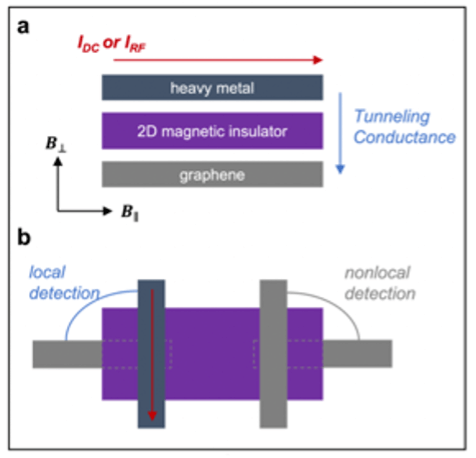
Coherent magnon generation, magnon condensation, and quantum spin liquids via spin pumping in 2D magnets
Summary Developing hybrid quantum systems is essential to harnessing the complementary advantages of different quantum technology platforms. This necessitates the successful transfer of quantum information between platforms, which can be achieved, e.g., by harnessing magnons, or spin wave excitations, in magnetic materials. Decoherence due to uncontrolled coupling of qubits to the environment remains a fundamental […]
February 1, 2023

